Comparative Effectiveness of Different Osteoporosis Medications in Enhancing Bone Mass
DOI:
https://doi.org/10.56929/jseaortho-2025-0231Keywords:
Osteoporosis, anti-resorptive agents, bone-forming agents, bone mineral density, bisphosphonates, denosumab, teriparatideAbstract
Purpose: To compare the spine and non-dominant hip bone mineral density before and after treatment with different categories of osteoporosis medications.
Methods: In this retrospective cohort study, we analyzed the medical records of patients with osteoporosis who were prescribed anti-resorptive agents (bisphosphonates, alendronate, risedronate, intravenous ibandronate, and denosumab) or bone-forming agents (teriparatide). Patients were selected using purposive sampling. Descriptive statistical analysis was performed, including calculations of percentages, means, and standard deviations, along with hypothesis testing using Wilcoxon signed-rank and t-tests.
Results: Among the 80 participants treated with these medications and monitored over 3–5 years, with at least 2 years of continuous treatment, none had hip or spine fractures. In the bisphosphonate group (n = 59), both the spine and non-dominant hip bone mineral density showed significant improvements. The denosumab group (n = 17) demonstrated a significant increase in spine bone mineral density, whereas the increase in nondominant hip bone mineral density was not significant. The teriparatide group (n = 4) showed improvements in both the spine and non-dominant hip bone mineral density, although not significant, possibly because of the small sample size.
Conclusions: All medication categories had positive effects on bone mineral density. Antiresorptive agents, particularly bisphosphonates, showed significant improvements in both spine and hip bone mineral density, whereas denosumab showed significant improvement, specifically in spine bone mineral density. The bone-forming agent teriparatide showed a positive trend, although not significant, likely because of the limited sample size.
Metrics
References
Leweicki EM, Watts NB. Assessing response to osteoporosis therapy. Osteoporos Int 2008;19:1363-8 DOI: https://doi.org/10.1007/s00198-008-0661-8
National Osteoporosis Foundation 2021. Physician’s guide to prevention and treatment of osteoporosis 2021. Available at: http: www.nof.org/. Accessed June 25, 2006.
Taechakraichana N, Angkawanich P, Panyakhamlerd K. Postmenopausal osteoporosis: what is the real magnitude of the problem in the Thai population? J Med Assoc Thai 1998;81:397-401.
Pongchaiyakul C, Apinyanurag C, Soontrapa S, et al. Prevalence of osteoporosis in Thai men. J Med Assoc Thai 2006;89:160-9.
The Bureau of Registration Administration Department of Provincial Administration (2022). Provincial population statistics management information system, January 2022. From https://stat.bora.dopa.go.th/StatMIS/#/ReportStat/3 (accessed on December 1, 2022). (In Thai)
Koh LK, Sedrine WB, Torralba TP, et al. A simple tool to identify asian women at increased risk of osteoporosis. Osteoporos Int 2001;12:699-705. DOI: https://doi.org/10.1007/s001980170070
Punichkul S, Sripramote M, Sriussawaamorn N. Diagnostic performance of quantitative ultrasound calcaneus measurement in case finding for osteoporosis in Thai postmenopausal women. J Obstet Gynaecol Res 2004;30:418-26. DOI: https://doi.org/10.1111/j.1447-0756.2004.00224.x
Grampp S, Genant HK, Mathur A, et al. Comparisons of noninvasive bone mineral measurements in assessing age-related loss, fracture discrimination, and diagnostic classification. J Bone Miner Res 1997;12:697-711. DOI: https://doi.org/10.1359/jbmr.1997.12.5.697
World Health Organization. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. No843 of technical reports series. Geneva: WHO; 1994.
Kanis JA, Johnell O, Oden A, et al. Ten year probabilities of osteoporotic fractures according to BMD and diagnostic thresholds. Osteoporos Int 2001;12:989-95. DOI: https://doi.org/10.1007/s001980170006
Lim JK, Lee JH, Kim JS, et al. Comparison of World Health Organization and Asia-Pacific body mass index classifications in COPD patients. Int J Chron Obstruct Pulmon Dis 2017:12:2465-75.
Black DM, Cumming SR, Katpf DB, et al. Randomized trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture intervention trial research group. Lancet 1996;348:1535-41. DOI: https://doi.org/10.1016/S0140-6736(96)07088-2
Cummings SR, Black DM, Thompson DE, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA 1998;280:2077-82. DOI: https://doi.org/10.1001/jama.280.24.2077
Sorensen OH, Crawford GM, Mulder H, et al. Long-term efficacy of risedronate: a 5-year placebo-controlled clinical experience. Bone 2003;32:120-6. DOI: https://doi.org/10.1016/S8756-3282(02)00946-8
Heaney RP, Zizic TM, Fogelman I, et al. Risedronate reduces the risk of first vertebral fracture in osteoporotic women. Osteoporos Int 2002;13:501-5. DOI: https://doi.org/10.1007/s001980200061
Bianchi G, Czerwinski E, Kenwright A, et al. Long-term administration of quarterly IV ibandronate is effective and well tolerated in postmenopausal osteoporosis: 5-year data from the DIVA study long-term extension. Osteoporos Int 2012;23:1769-78. DOI: https://doi.org/10.1007/s00198-011-1793-9
Delmas PD, Adami S, Strugala C, et al. Intravenous ibandronate injections in postmenopausal women with osteoporosis: one-year results from the dosing intravenous administration study. Arthritis Rheum 2006;54:1838-46. DOI: https://doi.org/10.1002/art.21918
Miller PD, Bolognese MA, Lewiecki EM, et al. Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomized blinded phase 2 clinical trial. Bone 2008;43:222-9. DOI: https://doi.org/10.1016/j.bone.2008.04.007
Ringe JD, Farahmand P. Improved real-life adherence of 6-monthly denosumab injections due to positive feedback based on rapid 6-month BMD increase and good safety profile. Rheumatol Int 2014;34:727-32. DOI: https://doi.org/10.1007/s00296-012-2663-2
Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 2001;344:1434-41. DOI: https://doi.org/10.1056/NEJM200105103441904
Langdahl BL, Ljunggren O, Benhamou CL, et al. Fracture rate, quality of life and back pain in patients with osteoporosis treated with teriparatide: 24-month results from the extended forsteo observational study (ExFOS). Calcif Tissue Int 2016;99:259-71. DOI: https://doi.org/10.1007/s00223-016-0143-5
Riggs BL, Melton Iii 3rd LJ, Rob RA, et al. Population-based study of age and sex differences in bone volumetric density, size, geometry, and structure at different skeletal sites. J Bone Miner Res 2004;19:1945-54. DOI: https://doi.org/10.1359/jbmr.040916
Khosla S, Riggs BL, Rob RA, et al. Relationship of volumetric bone density and structural parameters at different skeletal sites to sex steroid levels in women. J clin Endocorinal Metab 2005;90:5096-103. DOI: https://doi.org/10.1210/jc.2005-0396
Lim JU, Lee JH, Kim JS, et al. Comparison of World Health Organization and Asia-Pacific body mass index classifications in COPD patients. Int J Chron Obstruct Pulmon Dis 2017:12:2465-75. DOI: https://doi.org/10.2147/COPD.S141295
Robbins JA, Schott AM, Garmero P, et al. Risk factors for hip fracture in women with high BMD: EPIDOS study. Osteoporos Int 2005:16:149-54. DOI: https://doi.org/10.1007/s00198-004-1661-y
Silverman SL, Cummings SR. Watts NB. Recommendations for the clinical evaluation of agents for treatment of osteoporosis: consensus of an expert panel representing the American Society for Bone and Mineral Research (ASBMR), the International Society for Clinical Densitometry (ISCD), and the National Osteoporosis Foundation (NOF). J Bone Miner Res 2008;23:159-65. DOI: https://doi.org/10.1359/jbmr.070905
Watts NB. Treatment of osteoporosis with bisphosphonates. Endocrinol Metab Clin North Am 1998;27:419-39. DOI: https://doi.org/10.1016/S0889-8529(05)70014-1
Fast DK, Felix R, Dowse C, et al. The effects of diphosphonates on the growth and glycolysis of connective-tissue cells in culture. Biochem J 1978;172:97-107. DOI: https://doi.org/10.1042/bj1720097
Luckman SP, Hughes DE, Coxon FP, et al. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J Bone Miner Res 1998;13:581-9. DOI: https://doi.org/10.1359/jbmr.1998.13.4.581
Roger MJ, Crockett JC, Coxon FP, et al. Biochemical and molecular mechanisms of action of bisphosphonates. Bone 2011;49:34-41. DOI: https://doi.org/10.1016/j.bone.2010.11.008
Cranney A, Wells G, Willan A, et al. Meta-analyses of therapies for postmenopausal osteoporosis. II. Meta-analysis of alendronate for the treatment of postmenopausal women. Endocr Rev 2002;23:508-16. DOI: https://doi.org/10.1210/er.2001-2002
Papapoulos SE, Quandt SA, Liberman UA, et al. Meta-analysis of the efficacy of alendronate for the prevention of hip fractures in postmenopausal women. Osteoporos Int 2005;16:468-74. DOI: https://doi.org/10.1007/s00198-004-1725-z
Cranney A, Tugwell P, Adachi J, et al. Meta-analyses of therapies for postmenopausal osteoporosis. III. Meta-analysis of risedronate for the treatment of postmenopausal osteoporosis. Endroc Rev 2002;23:517-23. DOI: https://doi.org/10.1210/er.2001-3002
Wells GA, Hsieh SC, Zheng C, et al. Risedronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Database Syst Rev 2022;5:CD004523. DOI: https://doi.org/10.1002/14651858.CD004523.pub4
Recker R, Stakkestad JA, Chesnut 3rd CH, et al. Insufficiently dosed intravenous ibandronate injections are associated with suboptimal antifracture efficacy in postmenopausal osteoporosis. Bone 2004;34:890-9. DOI: https://doi.org/10.1016/j.bone.2004.01.008
Cranney A, Wells GA, Yetisir E, et al. Ibandronate for the prevention of nonvertebral fractures: a pooled analysis of individual patient data. Osteoporos Int 2009;20:291-7. DOI: https://doi.org/10.1007/s00198-008-0653-8
McClung MR, Lewiecki EM, Geller ML, et al. Effect of denosumab on bone mineral density and biochemical markers of bone turnover: 8-year results of a phase 2 clinical trial. Osteoporosis Int 2013;24:227-35 DOI: https://doi.org/10.1007/s00198-012-2052-4
Cummings SR, Martin JS, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 2009;361:756-65. DOI: https://doi.org/10.1056/NEJMoa0809493
McCloskey EV, Johansson H, Oden A, et al. Denosumab reduces the risk of osteoporotic fractures in postmenopausal women, particularly in those with moderate to high fracture risk as assessed with FRAX. J Bone Miner Res 2012;27:1480-6. DOI: https://doi.org/10.1002/jbmr.1606
Black DM, Greenspan SL, Ensrud KE, et al. The effect of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med 2003;349:1207-15. DOI: https://doi.org/10.1056/NEJMoa031975
Finkelstein JS, Hayes A, Hunzelman JL, et al. The effects of parathyroid hormone, alendronate or both in men with osteoporosis. N Engl J Med 2003;349:1216-26. DOI: https://doi.org/10.1056/NEJMoa035725
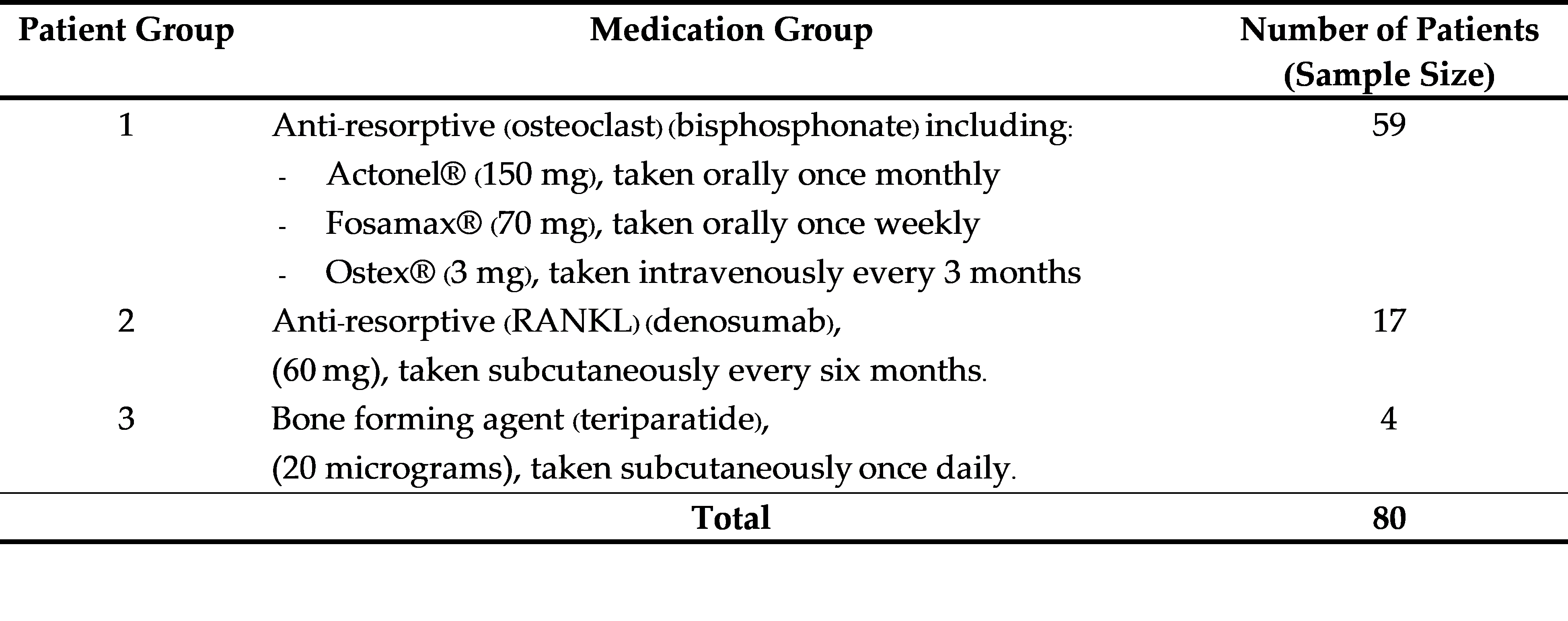
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 The Royal College of Orthopaedic Surgeons of Thailand

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






